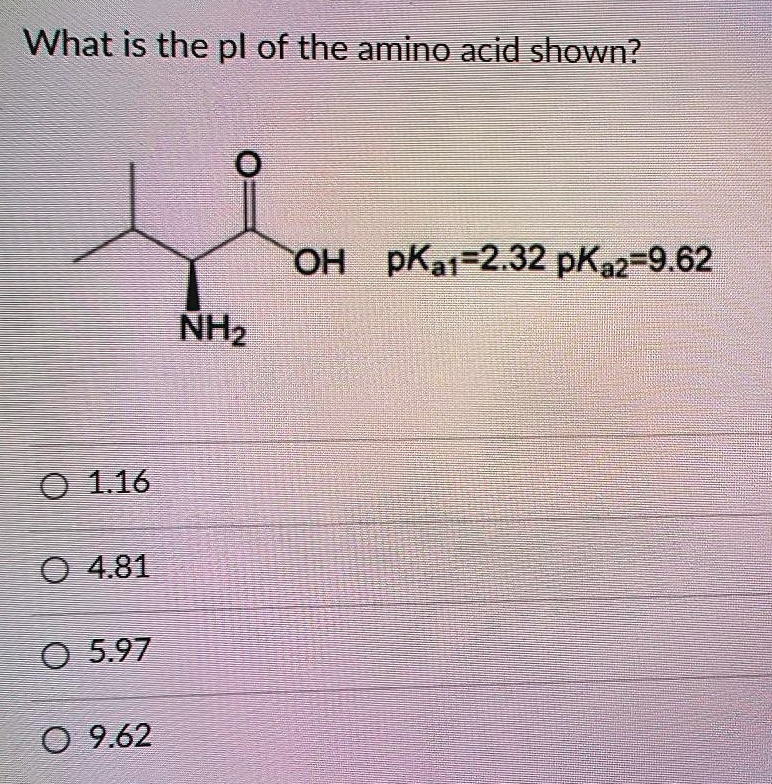
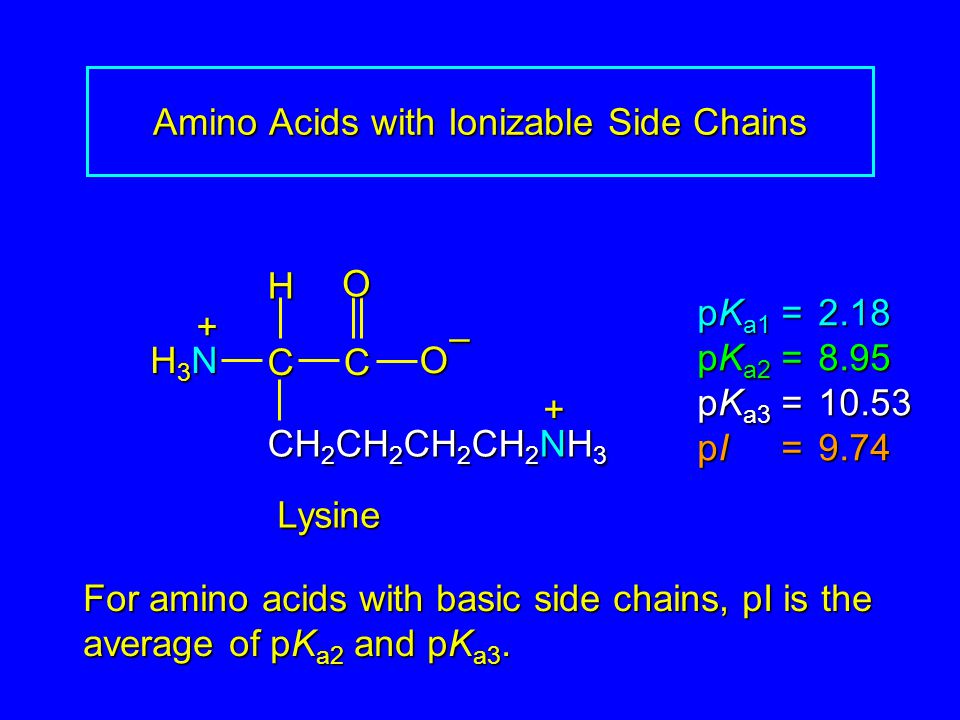
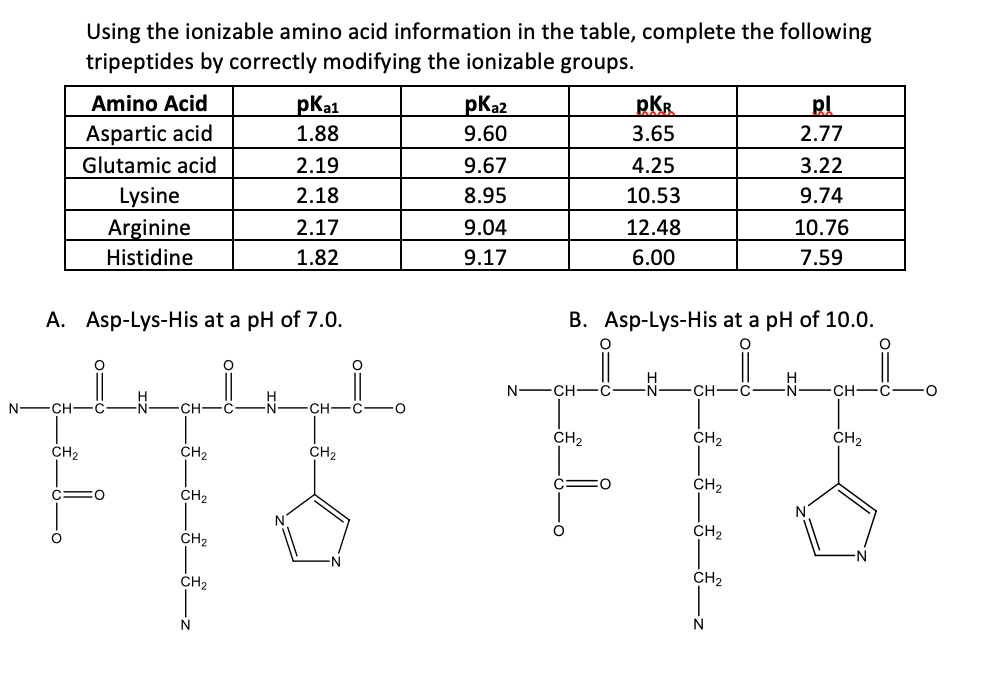
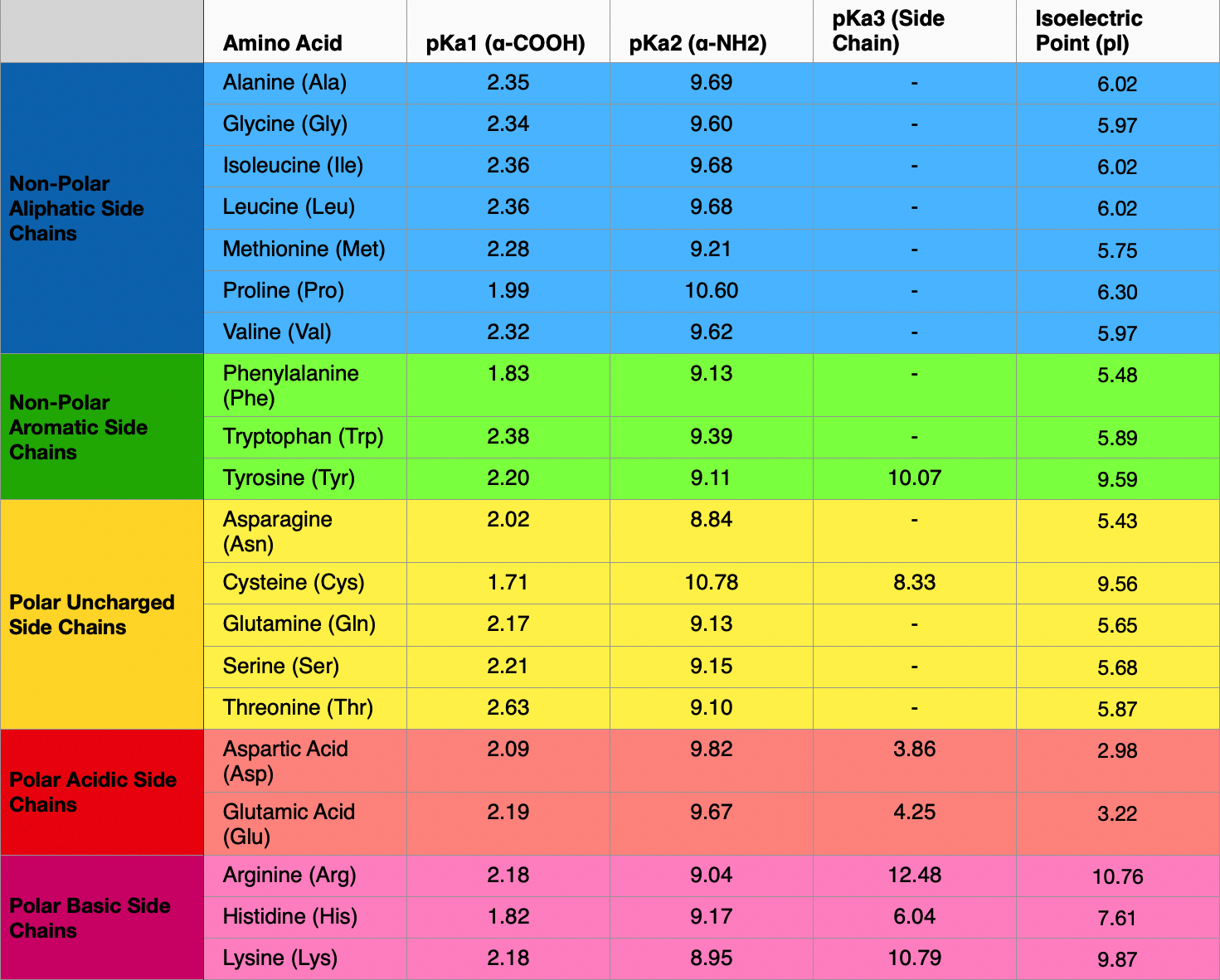
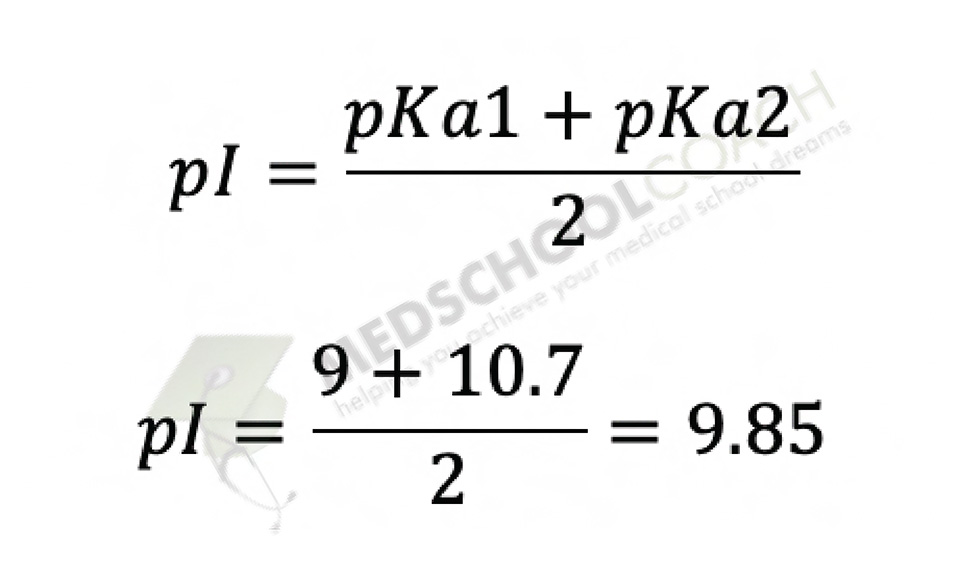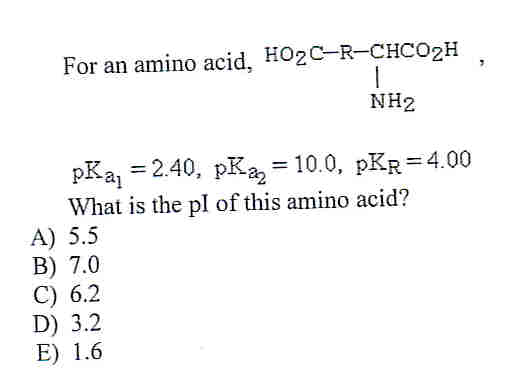Lysine has pKa1 = 2.18, pKa2 = 8.95, pKa3 = 10.53.In which structure lysine will be present at pH = 9.7.
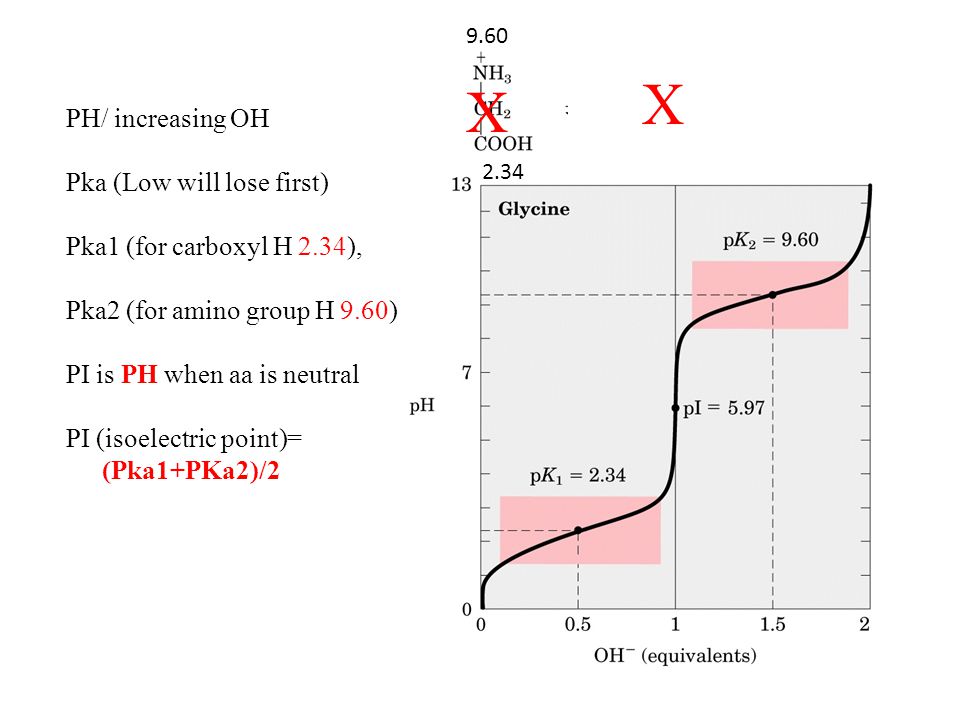
H OH - 2 Charge: +1 Charge: 0 (When aa have a net charged of zero its called a Zwitterion ) Charge: -1 Low PHHigh PH Adding a base PH=1 PH=7 PH=12 Pka= ppt download
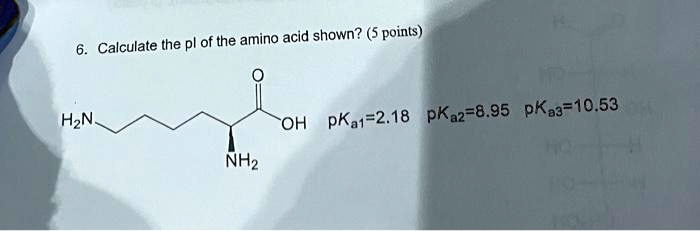
SOLVED: Calculate the pI of the amino acid shown. (5 points) H2N OH pKa1 = 2.18 pKa2 = 8.95 pKa3 = 10.53 NH2
Lecture 16: Polyprotic Acids We should be pretty comfortable dealing with monoprotic acids like: HCl HNO3 HClO4 CH3COOH +HN

The structure of aspartic acid is given below: NH (HOOC CH-CH2COOH) (A) The pKa,,pka, and pKaz of (A) respectively, are 1.88, 3.65 and 9.60. pka, and pka2 corresponds to the ionization of

Consider the following ionic equilibriumGiven pKa1 =2.3 and pKa2 =9.7Then what is the isoelectric point of alanine?