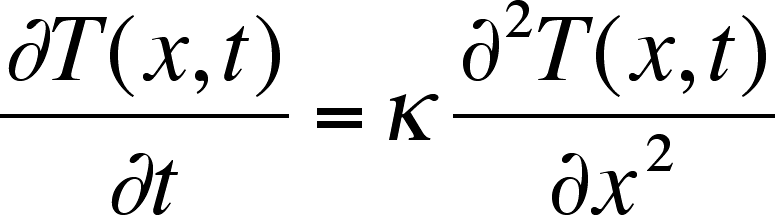Applied Sciences | Free Full-Text | A Novel Method for Calculating Diffusion Coefficient of Shale Gas Reservoirs: A Case Study of Longmaxi Formation in Weiyuan Area, Sichuan Basin, China
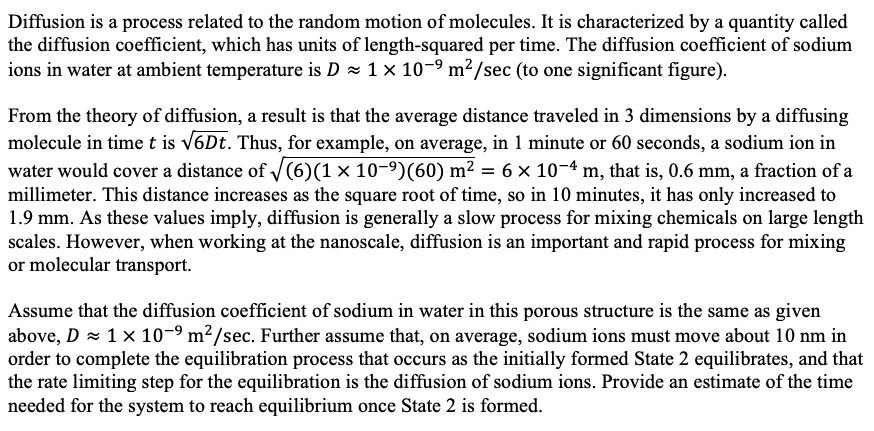
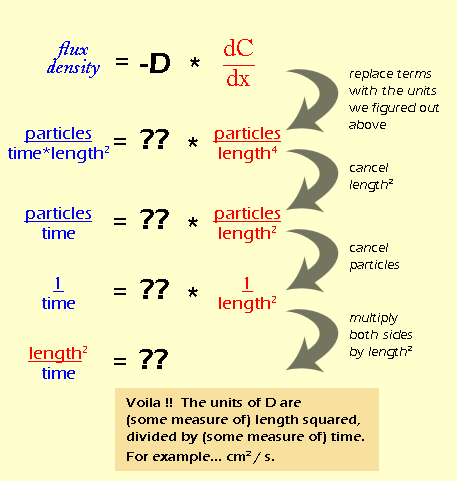
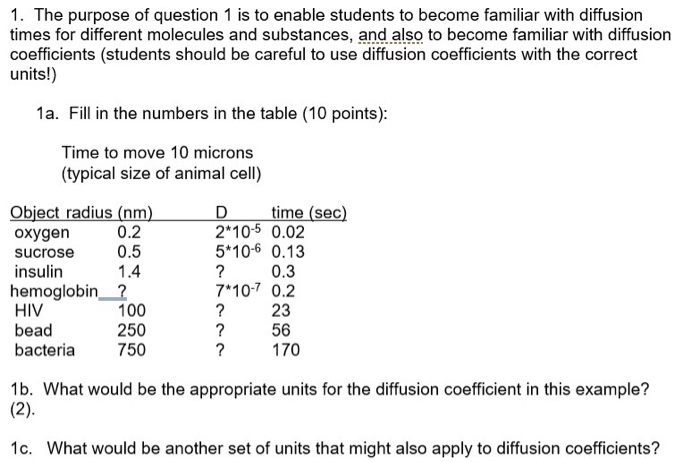
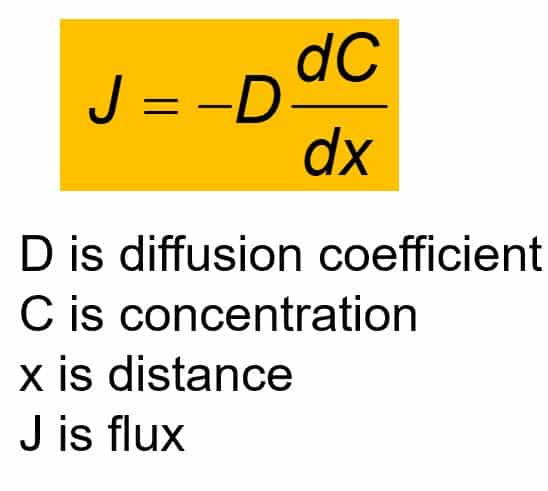
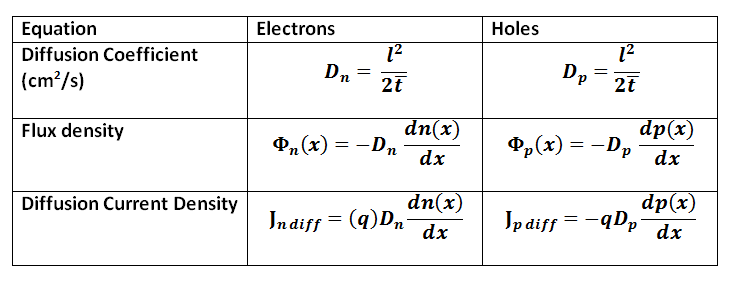
SOLVED: Diffusion is a process related to the random motion of molecules. It is characterized by a quantity called the diffusion coefficient, which has units of length-squared per time. The diffusion coefficient

Chapter ISSUES TO ADDRESS... How does diffusion occur? Why is it an important part of processing? How can the rate of diffusion be predicted for. - ppt download
![SOLVED: The diffusivity coefficient of a substance can be calculated from Fick's Law and is represented in the following equation: D = Dexp(1.987) D = diffusion coefficient, [cm/s] Do = maximum diffusion SOLVED: The diffusivity coefficient of a substance can be calculated from Fick's Law and is represented in the following equation: D = Dexp(1.987) D = diffusion coefficient, [cm/s] Do = maximum diffusion](https://cdn.numerade.com/ask_images/44fc79c0066242d5937b161df6d6d98c.jpg)
SOLVED: The diffusivity coefficient of a substance can be calculated from Fick's Law and is represented in the following equation: D = Dexp(1.987) D = diffusion coefficient, [cm/s] Do = maximum diffusion

The momentum diffusion coefficient, in units of classical diffusion... | Download Scientific Diagram